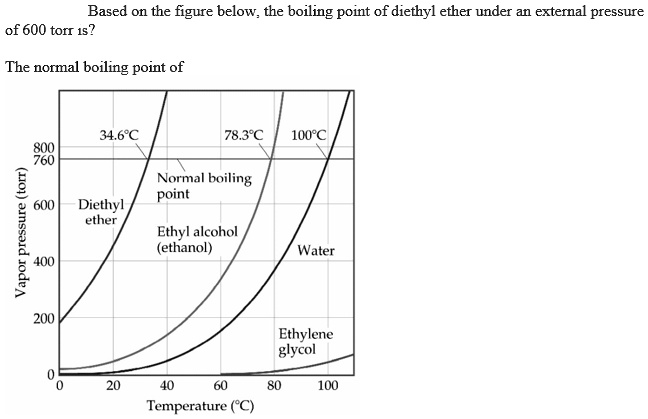
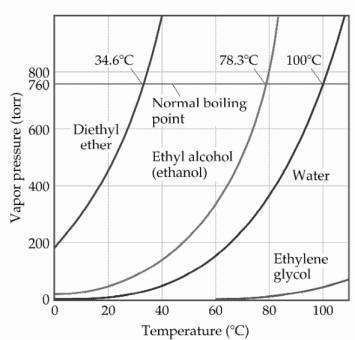
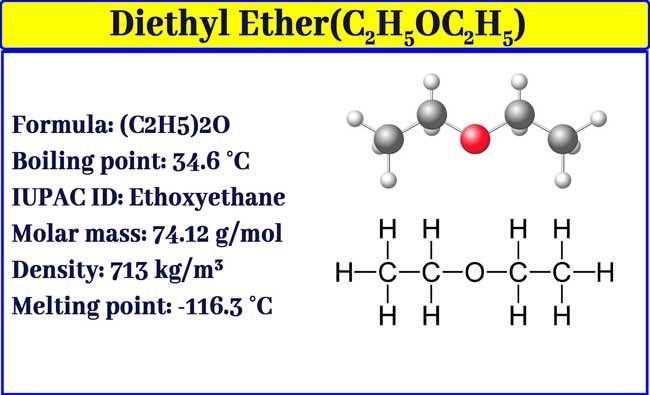
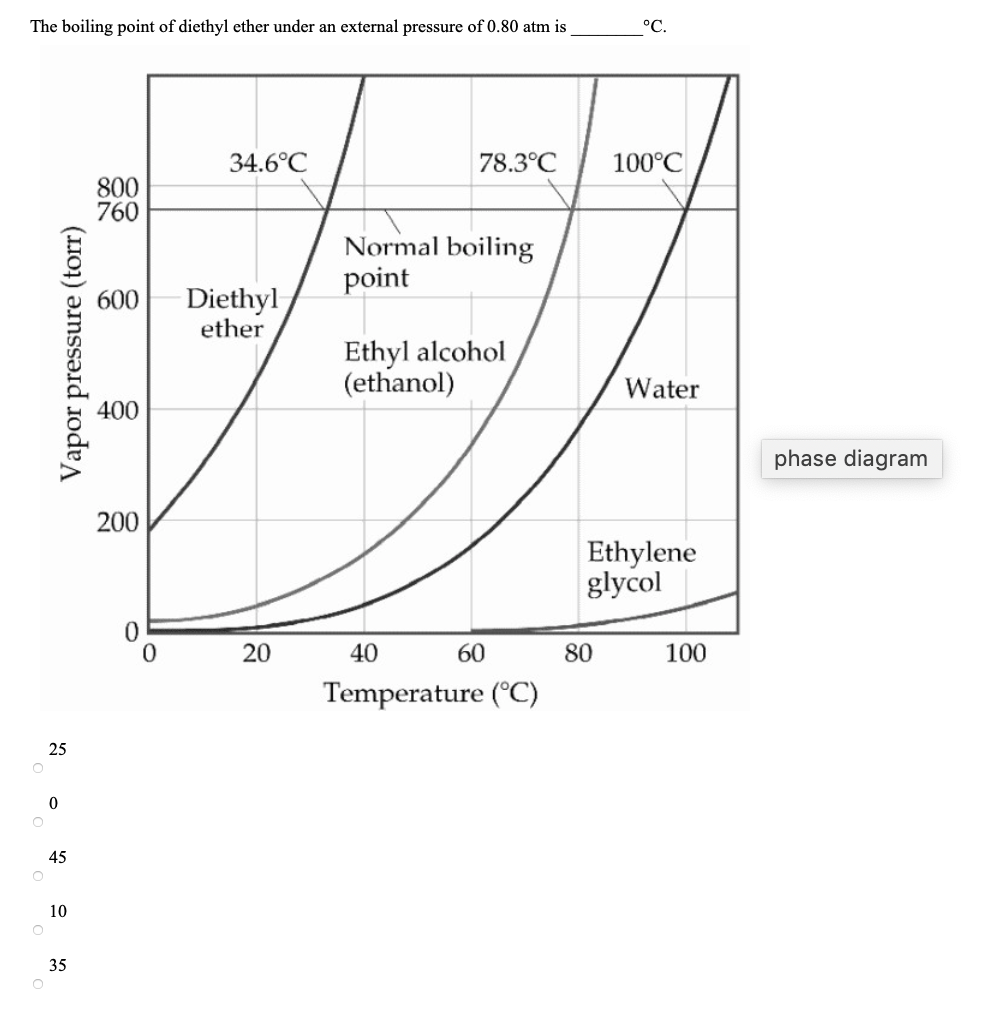
The boiling points of water, ethyl alcohol and diethyl ether are 100^∘C, 78.5^∘C and 34.6^∘C respectively. The intermolecular forces will be in the order of:
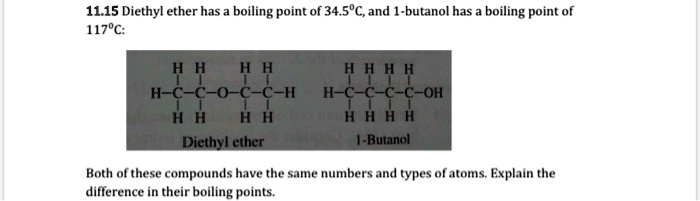
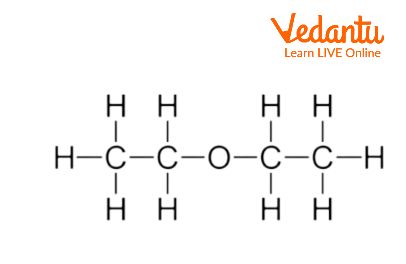
SOLVED: 11.15 Diethyl ether has a boiling point of 34.5°C, and butanol has a boiling point of 117°C. H H H H HH H H H-C-C-O-C-C-H H H H H Diethyl ether

Diethyl ether has a normal boiling point of `35.0^(@)C` and has an entropy of vaporization of `84.4 - YouTube

Ethyl alcohol and dimethyl ether have the same composition by mass (52% carbon, 13% hydrogen, and 35% oxygen), yet the two have different melting points, boiling points, and solubilities in water. Explain
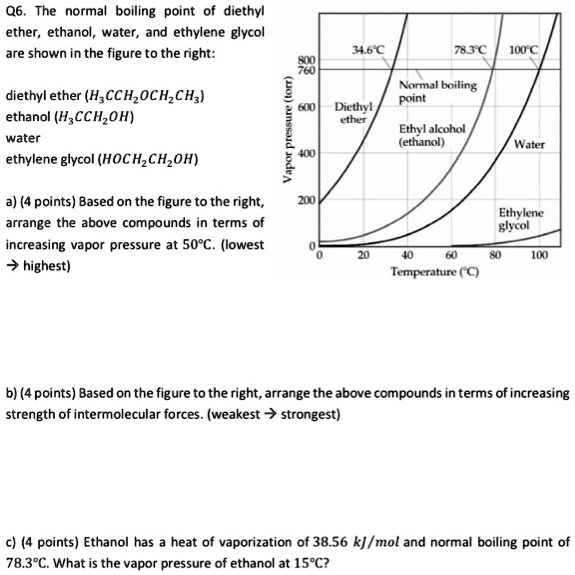
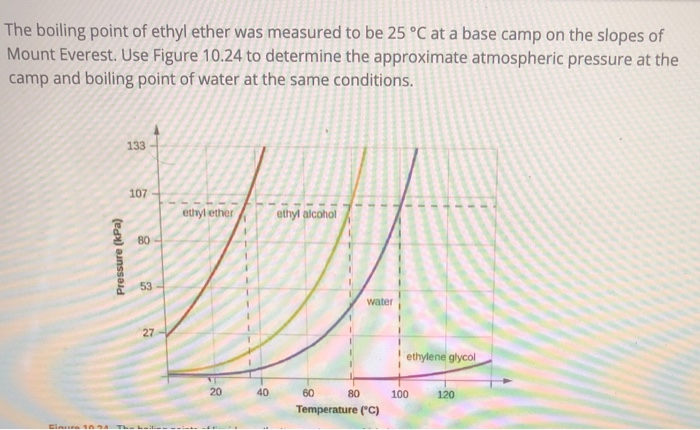
SOLVED: The normal boiling point of diethyl ether, ethanol, water, and ethylene glycol are shown in the figure to the right: diethyl ether (H3CCH2OCH2CH2) ethanol (C2H5OH) water ethylene glycol (HOCH2CH2OH) a) (4