How to Balance Fe+S=FeS|Chemical equation Fe+S=FeS|Fe+S=FeS balance equation| Reaction Fe+S=FeS - YouTube

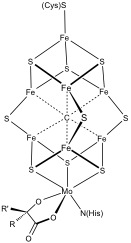
Fe-S cofactors in the SARS-CoV-2 RNA-dependent RNA polymerase are potential antiviral targets | Science

Strong 3D and 1D magnetism in hexagonal Fe-chalcogenides FeS and FeSe vs. weak magnetism in hexagonal FeTe | Scientific Reports

Assembly of Fe/S proteins in bacterial systems: Biochemistry of the bacterial ISC system - ScienceDirect

Mitochondrial iron–sulfur clusters: Structure, function, and an emerging role in vascular biology - ScienceDirect
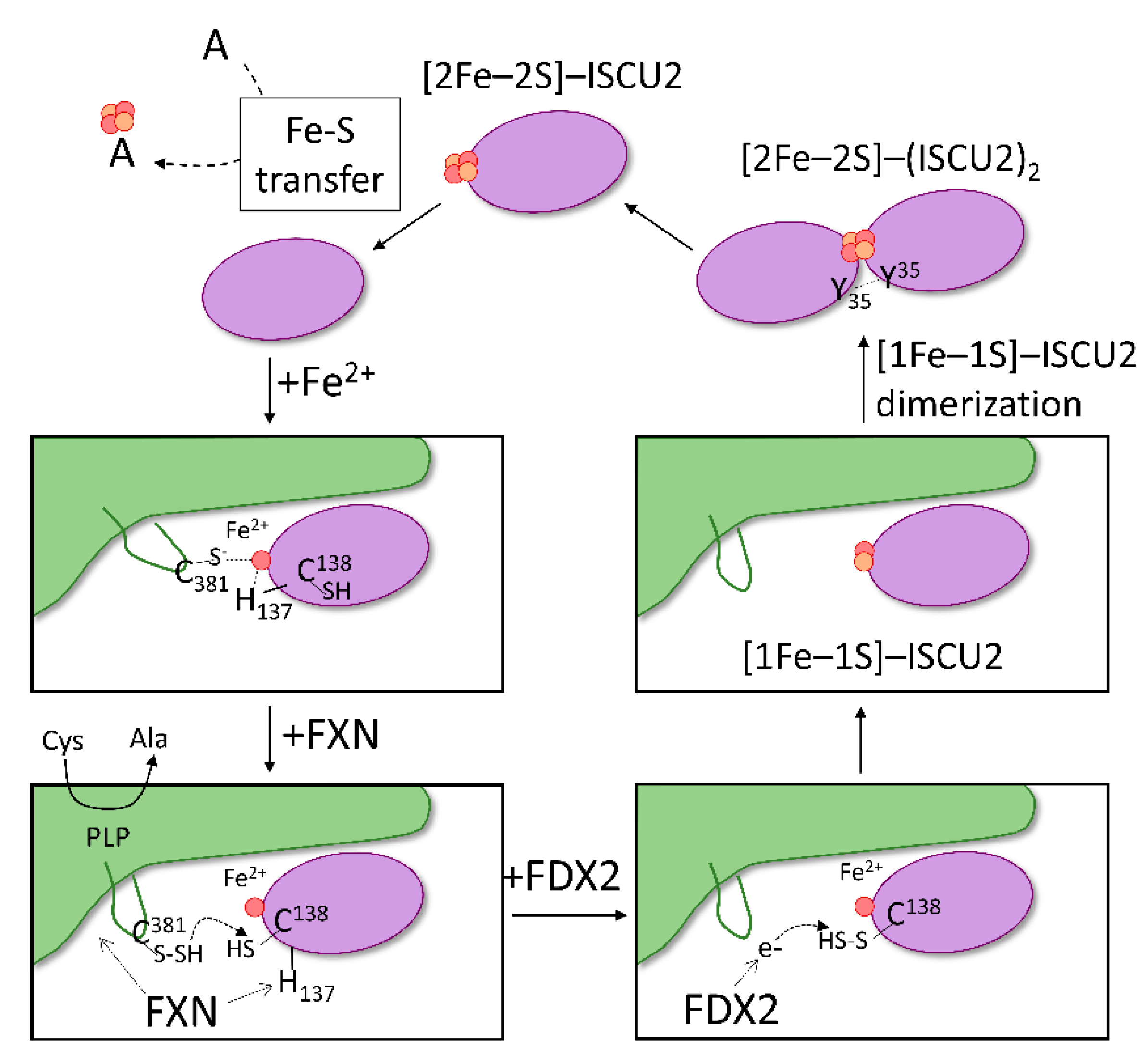
Inorganics | Free Full-Text | Mitochondrial De Novo Assembly of Iron–Sulfur Clusters in Mammals: Complex Matters in a Complex That Matters
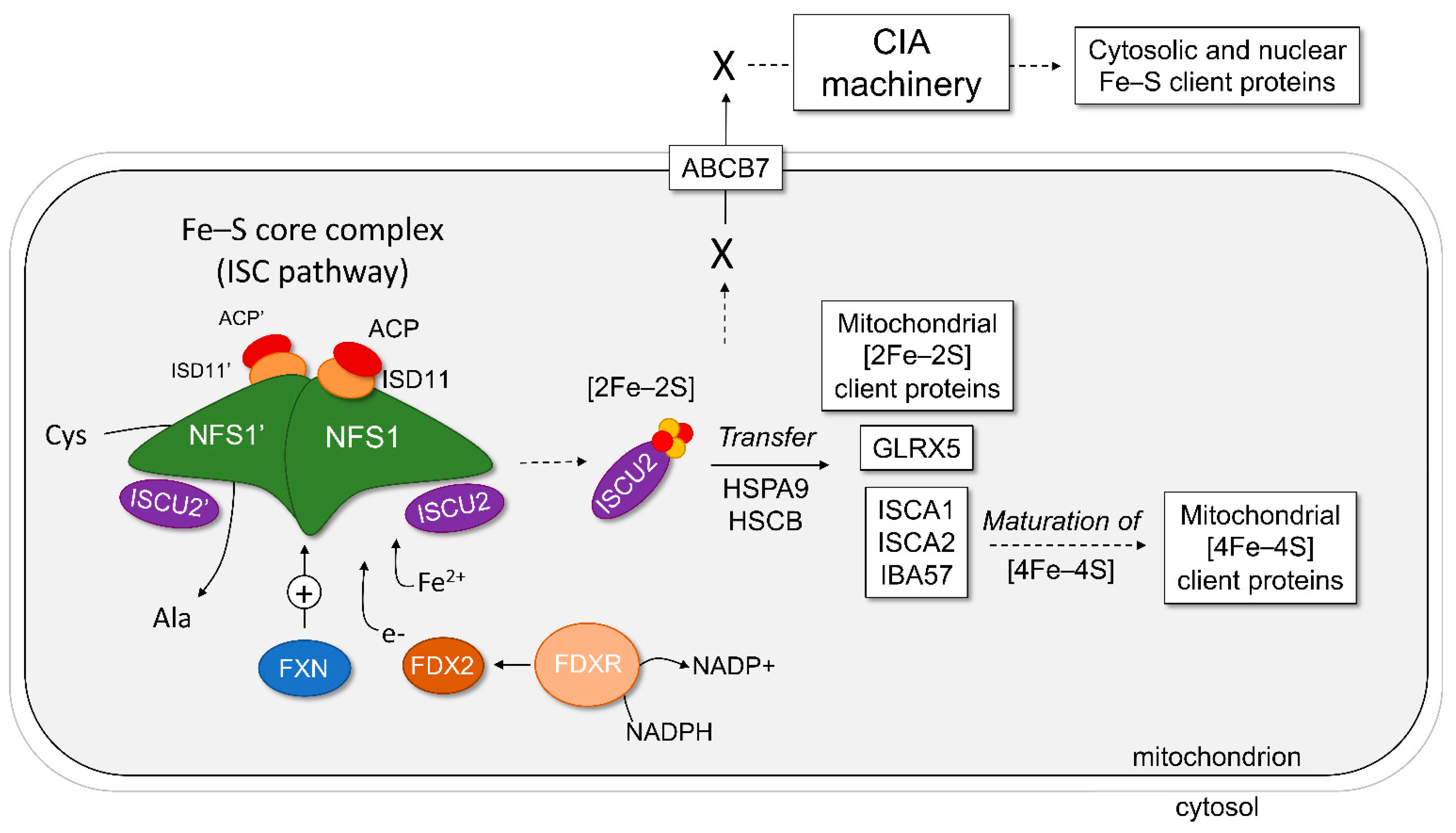
Inorganics | Free Full-Text | Mitochondrial De Novo Assembly of Iron–Sulfur Clusters in Mammals: Complex Matters in a Complex That Matters

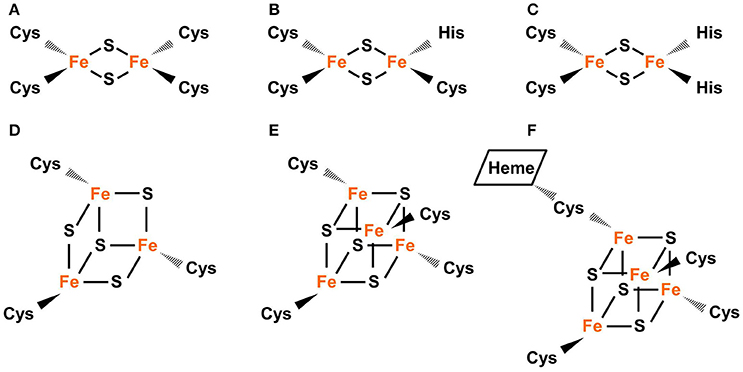
An iron-sulfur cube-shaped Fe 4 S 4 module. The Fe 4 S 4 center, as... | Download Scientific Diagram

Fe+S=FeS balance the chemical equation @mydocumentary838. fe+s=fes balance the chemical equation. - YouTube