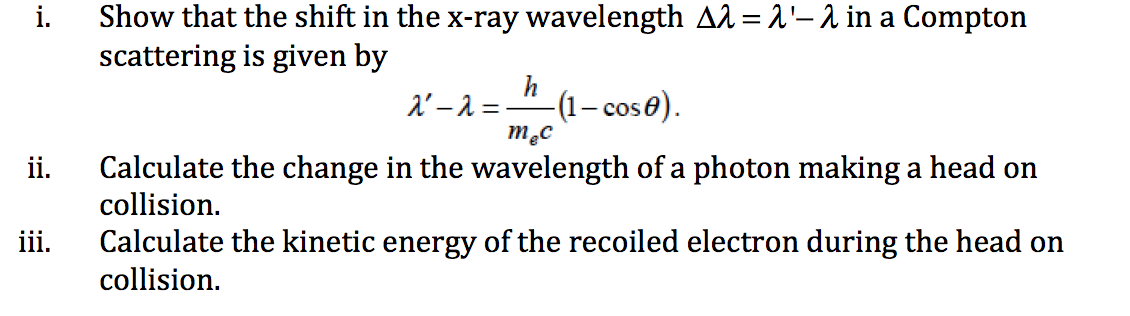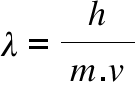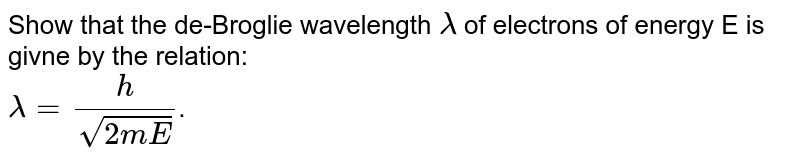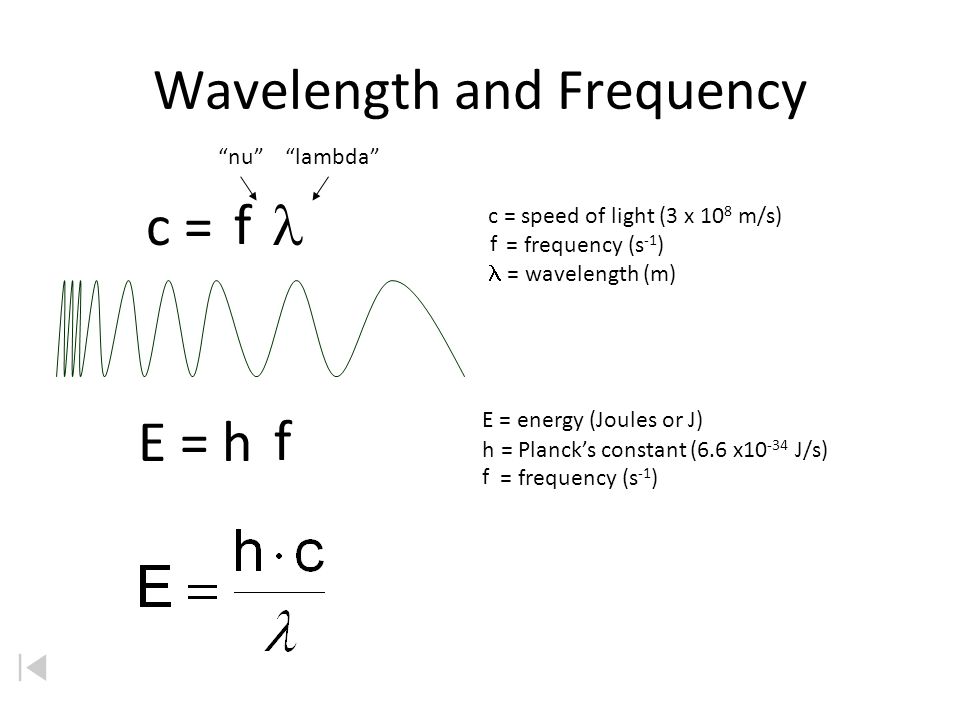
Wavelength and Frequency E = h c = c = speed of light (3 x 10 8 m/s) = frequency (s -1 ) = wavelength (m) E = energy (Joules or J) h = Planck's constant. - ppt download

Show that de Broglie wavelength lambda of electronsof energy E is given by the relation lambda=h+root2mE - Physics - Dual Nature Of Radiation And Matter - 14255610 | Meritnation.com
Sonda lambda 1.6 b z16xep opel astra h Second hand Vândut de firmă Cu garanție Horodnic de Jos • OLX.ro
68. A stationary atom of mass m emits a photon of wavelength lambda. The recoil speed of the atom is ?? In terms of planks constant,m and lambda ??
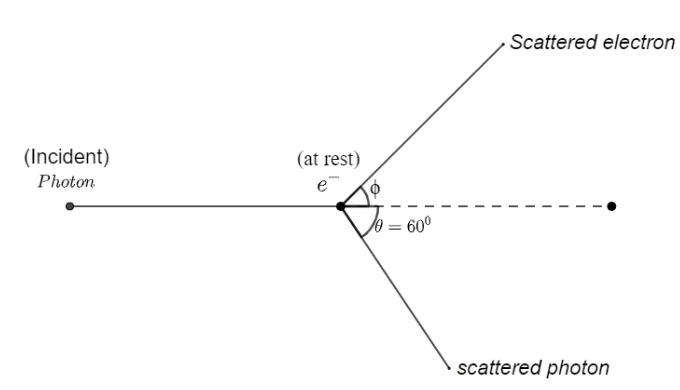
A photon of wavelength $\\lambda $ is scattered from an electron, which is at rest.The wavelength shift $\\Delta \\lambda $ is three times of $\\lambda $ and the angle of scattering $\\theta $

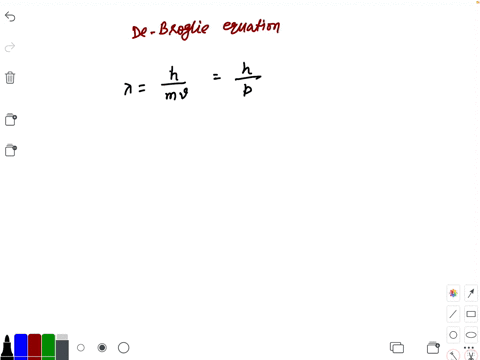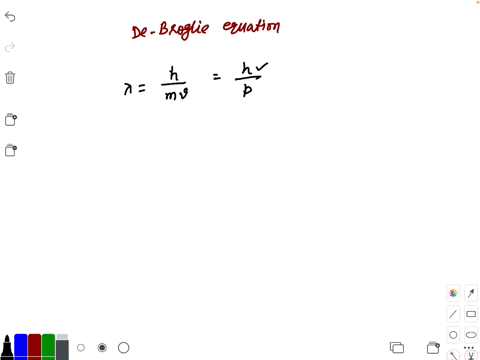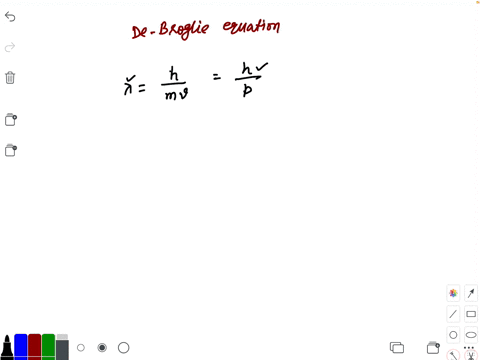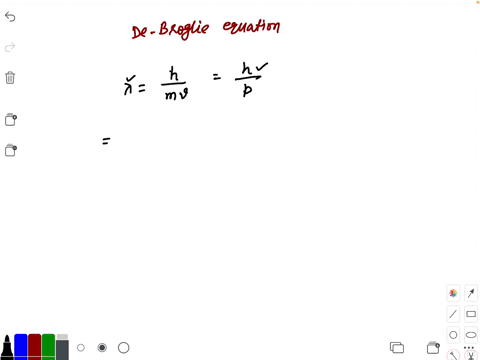
Prove dimensionally that lambda is proportional to h/m×v,where h is the Planck's constant,m is the mass and - Brainly.in


![The energy of photon of wavelength lambda is [h = Planck's constant, c = speed of light in vacuum] The energy of photon of wavelength lambda is [h = Planck's constant, c = speed of light in vacuum]](https://haygot.s3.amazonaws.com/questions/1412916_1014342_ans_a2202cd02f694985b93df75868250158.jpg)